In an era of increasing regulatory demands, the pharmaceutical industry faces the challenge of adhering to the Identification of Medicinal Products (IDMP) standards. This whitepaper provides guidance to understanding and implementing IDMP compliance, outlining the benefits and potential challenges, and offering practical solutions. We at AdEx Partners, as Trusted Leaders in Business Transformation, offer our expertise and proven methodologies to support enterprises in this complex journey.
Unlocking the Potential of IDMP Compliance
The IDMP standards, recently boosted by the European Medicines Agency (EMA) with their platform (Figure 1), aim to facilitate the reliable exchange of medicinal product information worldwide. Compliance with these standards is not just a regulatory requirement but a strategic move that can lead to improved patient safety, streamlined operations, and enhanced data management enabling your organization to see IDMP as a game changer, regardless of the regulatory environment. This can help position your organization better in several areas by providing a structured framework that improves communication between stakeholders and thereby enhances patient safety. Additionally, it can provide a competitive advantage because all departments speak “the same language” when it comes to data on medicinal products.
The Importance of IDMP Compliance
IDMP compliance is a significant step towards global data harmonization, enabling better communication between regulatory authorities, pharmaceutical companies, and healthcare providers. It ensures accurate identification of medicinal products, thereby reducing medication errors and enhancing patient safety. Moreover, it provides a structured framework for data, supporting efficient data management and analytics, so different areas profit from IDMP as follows:
Regulatory Affairs benefits from IDMP compliance by using a uniform standard for data capture and management, enhancing efficiency, and reducing errors in submissions. In Clinical Trials, IDMP forms agreed standards for data access and can improve medicinal product evaluations, as well as communication among stakeholders. Good Manufacturing Practice leverages IDMP for streamlined inspections and quicker detection of falsified medicines. In Pharmacovigilance, IDMP harmonizes product definitions to enhance data quality for risk identification and speeding up decision-making. Most importantly, non-compliance with (European) pharmacovigilance requirements based on IDMP standards can result in fines of up to 5% of annual revenue.
Challenges in Implementing IDMP Compliance
Implementing IDMP compliance is a complex task that requires a holistic approach. Challenges include reaching sufficient data quality, harmonization and literacy, technology integration, change management, and process re-engineering. Additionally, the lack of a clear roadmap and understanding of the standards can lead to inefficient implementation, potential gaps, and non-compliance. In addition, EMA is in the advanced stages of finalizing the implementation roadmap. While Figure 2 illustrates the presently communicated roadmap, it's important to note that it may be subject to further modifications in the near future. This potential for change introduces a degree of uncertainty, which makes it challenging to accurately forecast the specific requirements and their respective implementation deadlines. This uncertainty adds to the complexities of planning and impedes adhering to compliance.
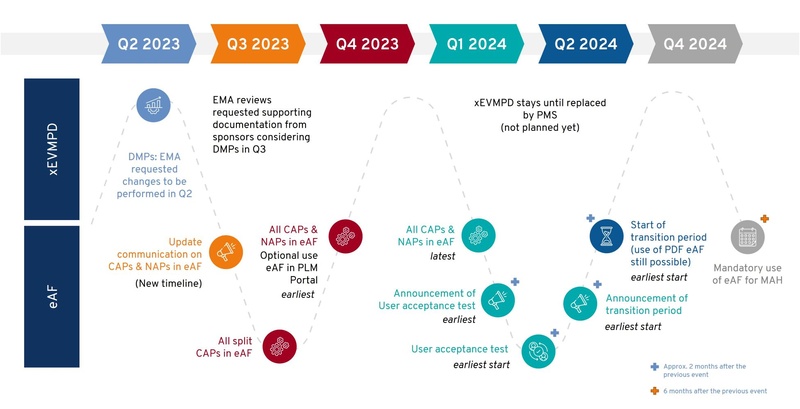
eAF: electronic Application Form (new eAF), xEVMPD: eXtended EudraVigilance Medicinal Product Dictionary,
DMP: Development medicinal product, CAP: Centrally authorized product, NAP: Nationally authorized product,
MAH: Marketing authorization holder,
Source: EMA webinars, https://esubmission.ema.europa.eu/cessp/202307_eAF%20Timeline.pdf
AdEx Partners: Your Trusted Guide for Achieving and Sustaining IDMP Compliance
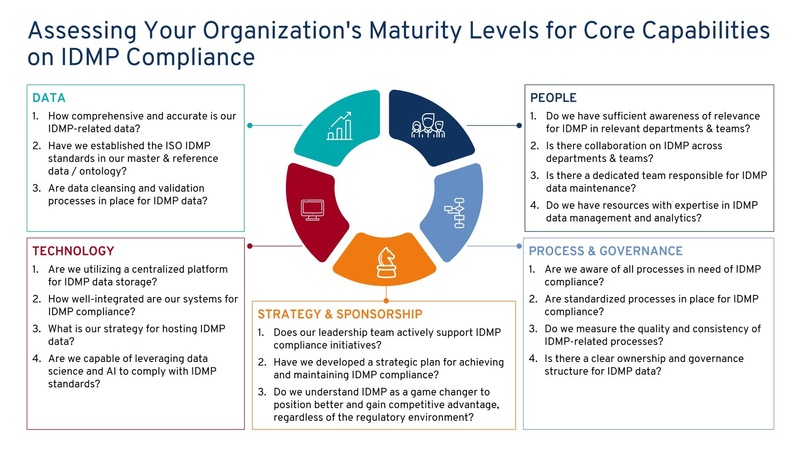
AdEx Partners offers a comprehensive solution approach to achieve and maintain IDMP compliance. Leveraging our deep industry knowledge and technical expertise, we help pharmaceutical companies navigate the complexities of IDMP compliance with our cross-functional team. Our services include maturity assessments (Figure 3), strategy development, technology selection, and implementation, including change management, process re-engineering, and ongoing support. At AdEx Partners, we employ a proactive approach to stay abreast of ongoing regulatory changes. Our strategies include active participation in regulatory forums, continuous tracking of regulatory updates, and leveraging our strong network within the industry. This allows us to consistently remain at the cutting edge, ensuring we are always in the know about the latest developments.
A Step-by-Step Guide to Implementing IDMP Compliance
- Strategy & Sponsorship: Develop a clear strategy for IDMP compliance. Ensure sponsorship from top management to ensure commitment and resource allocation.
- Data: Begin by assessing the quality and availability of your data. Establish processes for data governance and harmonization.
- People: Identify the key stakeholders and their roles. Implement a change management plan to support your team through the transition and enable them for data literacy.
- Technology: Choose the right technology solutions that align with your data and process requirements. Ensure seamless integration with existing systems.
- Process & Governance: Design and implement processes in line with IDMP standards. Establish a governance structure for ongoing compliance.
Together We Can Navigate the Complexities of IDMP Compliance
IDMP compliance is a complex but necessary journey for pharmaceutical companies. With the right approach and support, it can become an opportunity to enhance data management, improve patient safety, and streamline operations. AdEx Partners is committed to assisting pharmaceutical companies in this journey, providing expert guidance and proven solutions to achieve successful IDMP compliance.